Poster Presentation The 13th International Congress of the Immunology of Diabetes Society 2013
Efficient mail-based sampling for Type 1 diabetes (T1D) autoantibody surveillance (#83)
General population prediction of T1D before clinical onset prevents onset morbidity and identifies candidates for prevention therapies. After HLA screening, prediction involves repeated islet autoantibody (aab) surveillance of those at risk, requiring a cost-effective real-time sampling method. We tested an affordable mail-based kit for blood sampling at home or local doctor’s office. The kit includes a spring-loaded lancet, collection tube and return mailer. Total cost for materials and 2-way first class US postage was under $10.
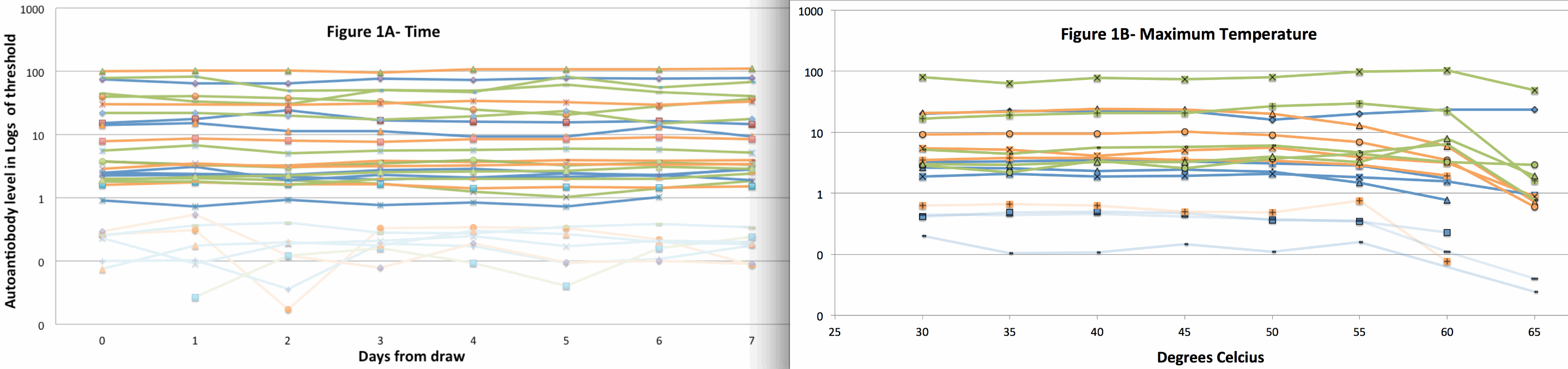
We first determined stability of aab to mailing conditions of time and temperature. We observed no change in aab titer for GADab, IA2ab nor IAA up to 7 days in the mail (n=22 samples, Fig 1A). Aab levels were stable at ≤55°C but most decreased markedly at 65°C (n=10 samples, Fig 1B).
We then mailed kits to individuals with elevated HLA risk of T1D for prospective finger-stick sampling. Of n=414 samples mailed back, the mean/median time from blood draw to lab receipt was 3.4/2.0 days. 392/414 (94.7%) samples were received ≤7 days after sampling. Kits contained temperature dot strips measuring maximum exposure. Mean/median maximum temperature were 38.1°C/37.0°C. Only 2/414 (0.5%) samples exceeded 53°C. After centrifugation, these 414 fingerstick kits yielded a mean/median of 193ul / 93ul of supernatant serum, with 341/414 (82.4%) >25ul sufficient to determine GADab, IA2ab and IAA levels in our laboratory (our aab assay details available in the IASP workshop). In a separate series, inclusion of an instructional DVD in the mailed kit increased serum volume yields to >25ul in 633/657 (96.3%) pediatric samples (mean age 12.8, age range 1.3-17.9). Total kit cost remained under $10.
We conclude that islet autoantibodies are stable to conditions of standard US mail, and a mail-based kit is useful for cost-effective collection of samples for pediatric islet aab surveillance.
- Funded by WA State Life Science Discovery Fund and by the US Dept of Defense.